Market Overview
The pediatric epilepsy therapeutic market is experiencing significant growth due to the rising incidence of epilepsy in children and advancements in anti-epileptic drugs (AEDs) and neurostimulation therapies. Pediatric epilepsy is a neurological disorder characterized by recurrent seizures and can significantly impact a child’s cognitive and developmental progress. The increasing adoption of personalized medicine, improved diagnostic techniques, and non-invasive treatment options is expected to drive market expansion.
Market Size and Share
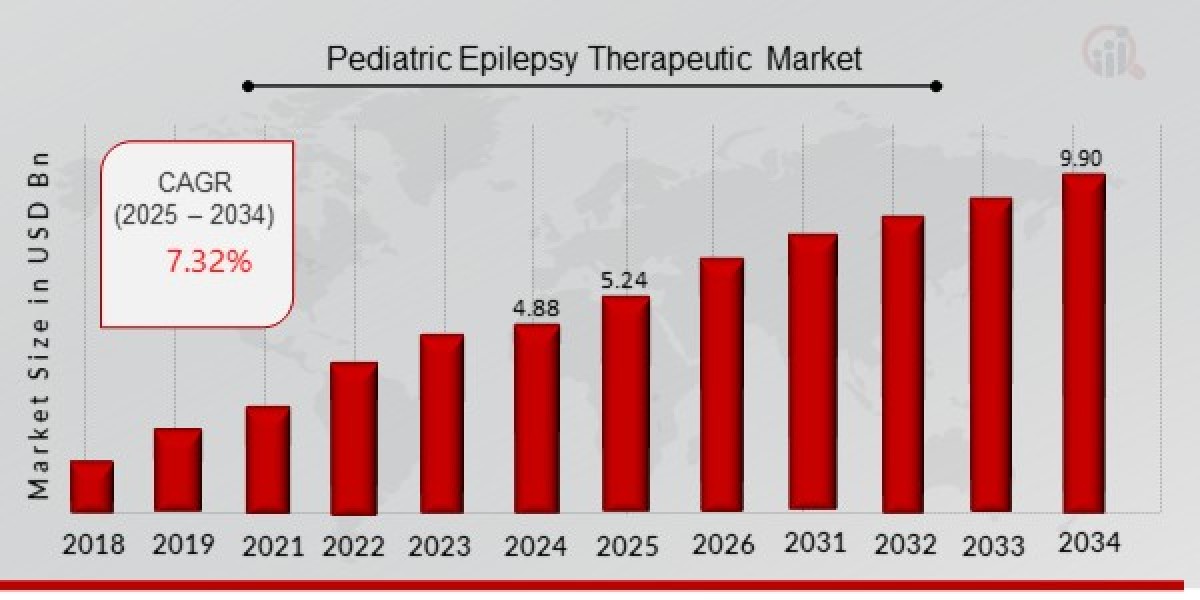
Pediatric Epilepsy Therapeutic Market Size was estimated at 4.88 (USD Billion) in 2024. The Pediatric Epilepsy Therapeutic Market Industry is expected to grow from 5.24 (USD Billion) in 2025 to 9.90 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 7.32% during the forecast period (2025 - 2034). The global pediatric epilepsy market is dominated by North America, followed by Europe, due to the high prevalence of epilepsy, well-established healthcare infrastructure, and availability of advanced treatment options. The Asia-Pacific region is expected to grow at a significant rate, driven by increasing awareness, improved healthcare accessibility, and a rising number of epilepsy treatment centers.
Market Trends
- Growing Use of Precision Medicine: Personalized treatment approaches based on genetic testing are becoming more common.
- Advancements in Neurostimulation Therapy: Non-invasive vagus nerve stimulation (VNS) and responsive neurostimulation (RNS) are being explored as alternatives to traditional AEDs.
- Development of Novel Anti-Seizure Medications: Pharmaceutical companies are introducing new drugs with fewer side effects.
- Increased Adoption of Telemedicine for Epilepsy Management: Virtual consultations and remote EEG monitoring are becoming more prevalent.
Growth Drivers
- Rising Prevalence of Pediatric Epilepsy: Increasing cases of epilepsy in infants and children are driving demand for improved treatment options.
- Growing Investment in Neurological Research: Governments and private organizations are funding epilepsy research to develop better therapies.
- Improved Access to Advanced Healthcare: Expansion of specialized epilepsy centers is improving access to care.
- Rising Awareness Campaigns: Educational initiatives are reducing stigma and promoting early diagnosis and treatment.
Challenges and Restraints
- High Cost of Advanced Therapies: New-generation AEDs and neurostimulation devices can be expensive.
- Limited Availability of Pediatric Neurologists: Many regions suffer from a shortage of specialists trained in pediatric epilepsy care.
- Side Effects of Anti-Epileptic Drugs: Some AEDs can cause cognitive impairment, behavioral changes, and other adverse effects.
Regional Analysis
- North America: Leads the market due to advanced medical technology, strong research funding, and established epilepsy programs.
- Europe: Growing market due to improved healthcare policies and access to innovative epilepsy treatments.
- Asia-Pacific: Expected to witness rapid growth due to rising healthcare investments, increasing awareness, and expanding pediatric care infrastructure.
Segmental Analysis
- By Treatment Type:
- Anti-Epileptic Drugs (AEDs)
- Ketogenic Diet Therapy
- Neurostimulation Devices
- Surgical Procedures
- By Seizure Type:
- Focal Seizures
- Generalized Seizures
- Epileptic Spasms
- By End-User:
- Hospitals
- Specialty Clinics
- Research Institutions
Key Market Players
- UCB Pharma
- Eisai Co., Ltd.
- Pfizer Inc.
- Neurelis, Inc.
- Greenwich Biosciences (Jazz Pharmaceuticals)
Recent Developments
- Launch of Next-Generation Anti-Seizure Medications: Companies are introducing new AEDs with better safety profiles.
- Growing Research on Gene Therapy for Epilepsy: Studies are exploring genetic-based treatments for drug-resistant epilepsy.
- Increased FDA Approvals for Pediatric Epilepsy Drugs: Regulatory agencies are accelerating approvals for novel epilepsy treatments.
For more information, please visit us at @marketresearchfuture.