Market Overview
The omics-based clinical trial market is experiencing rapid growth, driven by advancements in genomics, proteomics, transcriptomics, metabolomics, and epigenomics. These technologies enable precision medicine, biomarker discovery, and personalized drug development, improving clinical trial outcomes. Omics-based trials are transforming oncology, neurology, cardiology, and rare disease research by providing deeper molecular insights into disease mechanisms.
With the increasing adoption of next-generation sequencing (NGS), bioinformatics tools, and AI-driven data analysis, the market is set to expand significantly. Pharmaceutical and biotechnology companies are leveraging omics technologies to optimize drug development pipelines, reduce trial failures, and accelerate time-to-market.
Market Size and Growth Potential
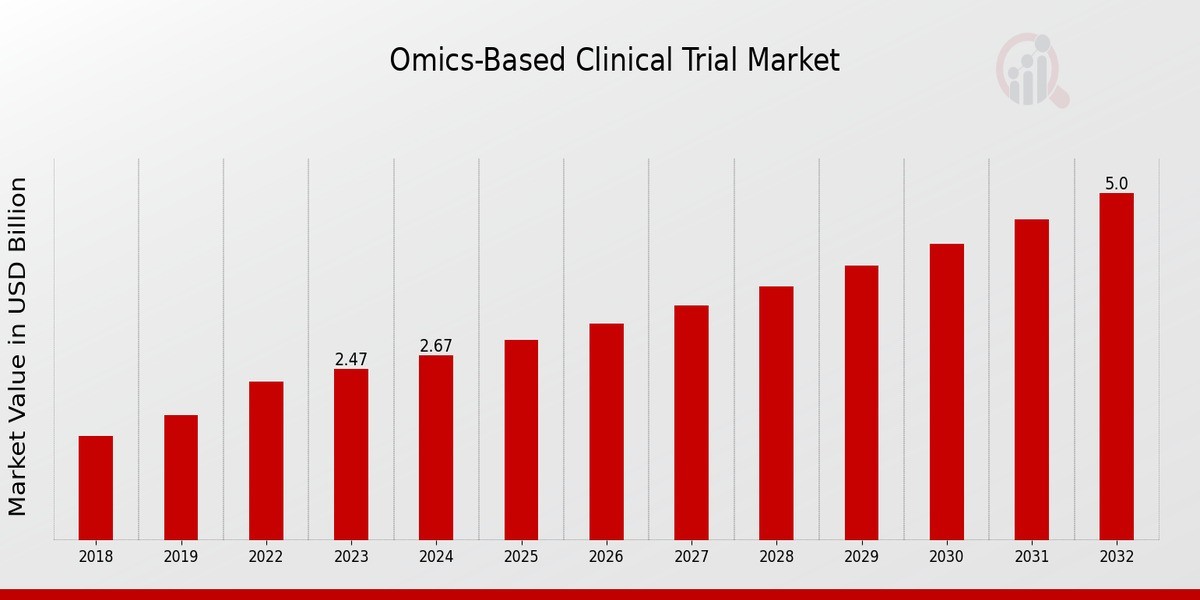
Omics Based Clinical Trial Market Size was estimated at 2.89 (USD Billion) in 2024. The Omics Based Clinical Trial Market Industry is expected to grow from 3.12 (USD Billion) in 2025 to 6.34 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 8.18% during the forecast period (2025 - 2034). The global omics-based clinical trial market is expected to grow steadily, driven by increasing investments in precision medicine and biomarker-driven drug development. North America leads in market share due to strong regulatory support and significant research funding, while Europe and Asia-Pacific are witnessing rapid adoption due to expanding genomic research infrastructure.
Market Trends and Key Developments
- Growing Adoption of Precision Medicine: Omics-based trials enable customized drug development by analyzing patient-specific molecular profiles.
- Integration of AI and Big Data Analytics: AI-driven algorithms are enhancing omics data interpretation and predictive analytics for clinical trials.
- Rise in Biomarker-Driven Drug Approvals: The FDA and EMA are increasingly approving drugs based on biomarker-driven trial designs.
- Expansion of Multi-Omics Approaches: Combining genomics, proteomics, and metabolomics is improving drug efficacy predictions.
Growth Drivers
- Increasing Demand for Personalized Medicine
- Omics-based trials are critical in developing targeted therapies for cancer, autoimmune diseases, and neurological disorders.
- Advancements in Genomic Technologies
- The affordability and accessibility of NGS and CRISPR-based gene editing are fueling the market's growth.
- Rising Investments in Biomarker Discovery
- Biomarkers play a crucial role in patient stratification and treatment response monitoring in clinical trials.
- Regulatory Support for Omics Integration
- Regulatory agencies are encouraging omics-based trial designs to improve drug approval success rates.
Challenges and Restraints
- Data Complexity and Standardization Issues: Managing large-scale omics data requires advanced bioinformatics infrastructure.
- High Costs Associated with Omics Technologies: The expenses for sequencing, proteomics analysis, and computational modeling can be substantial.
- Regulatory and Ethical Concerns: Ethical considerations regarding genomic data privacy and patient consent can impact trial designs.
Regional Analysis
- North America: Leads the market due to strong research funding, established clinical trial networks, and regulatory support.
- Europe: Countries like Germany, the UK, and France are investing in multi-omics research and translational medicine.
- Asia-Pacific: China, India, and Japan are emerging as key players due to growing genomic initiatives and increasing clinical trial outsourcing.
- Latin America & Middle East: Expanding biotech sectors and clinical research investments are boosting market opportunities.
Segmental Analysis
- By Technology:
- Genomics
- Proteomics
- Metabolomics
- Transcriptomics
- Epigenomics
- By Therapeutic Area:
- Oncology
- Neurology
- Cardiology
- Rare Diseases
- By End-User:
- Pharmaceutical & Biotech Companies
- Academic Research Institutes
- CROs (Contract Research Organizations)
Key Market Players
- Illumina, Inc.
- Thermo Fisher Scientific, Inc.
- Qiagen N.V.
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- PerkinElmer, Inc.
- Novartis AG
- Roche Holding AG
Recent Developments
- Advances in AI-Based Omics Data Interpretation: AI-powered platforms are improving biomarker discovery and clinical trial stratification.
- Strategic Collaborations for Multi-Omics Research: Pharma companies are partnering with academic research centers to develop omics-based therapeutics.
- Regulatory Approvals for Omics-Guided Drugs: Increasing FDA approvals for biomarker-driven therapies are reshaping the market landscape.
Future Outlook
The omics-based clinical trial market is set to grow with the increasing integration of genomics, AI, and personalized medicine in clinical research. Expanding applications in oncology, rare diseases, and regenerative medicine will further drive market expansion.
For more information, please visit us at @marketresearchfuture.