The medical device industry is one of the most heavily regulated sectors, with strict compliance requirements imposed by regulatory bodies such as the FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), and TGA (Therapeutic Goods Administration). Keeping up with evolving regulations, documentation, and approval processes can be overwhelming for medical device manufacturers. This has led to the growing trend of medical device regulatory affairs outsourcing as a strategic solution to ensure compliance while focusing on core innovations.
Why Outsource Medical Device Regulatory Affairs?
Outsourcing regulatory affairs offers numerous advantages, including:
- Expert Guidance on Regulations: Regulatory requirements frequently change, and specialized firms stay updated on the latest standards, ensuring that devices meet compliance.
- Faster Market Access: Skilled professionals expedite approval processes, reducing delays and helping companies launch their products sooner.
- Cost Efficiency: Maintaining an in-house regulatory team can be expensive. Outsourcing reduces overhead costs while ensuring high-quality regulatory compliance.
- Risk Mitigation: Non-compliance can lead to costly penalties and product recalls. Outsourced experts help mitigate these risks by ensuring thorough regulatory adherence.
- Global Market Expansion: Different regions have unique regulations. Outsourced regulatory affairs teams facilitate smooth market entry across multiple geographies.
Key Services Offered by Regulatory Affairs Outsourcing Firms
Regulatory affairs service providers assist medical device manufacturers through various stages, including:
- Regulatory Strategy Development: Crafting a compliance roadmap tailored to specific markets.
- Pre-Market Approval (PMA) and 510(k) Submissions: Ensuring proper documentation and submission to regulatory bodies.
- Clinical Evaluation Reports (CER) and Technical File Preparation: Preparing essential documents for EU MDR and FDA submissions.
- Post-Market Surveillance (PMS): Monitoring product safety and effectiveness post-launch.
- Regulatory Compliance Training: Providing staff training on the latest regulatory requirements.
Medical Device Regulatory Affairs Outsourcing Market Overview
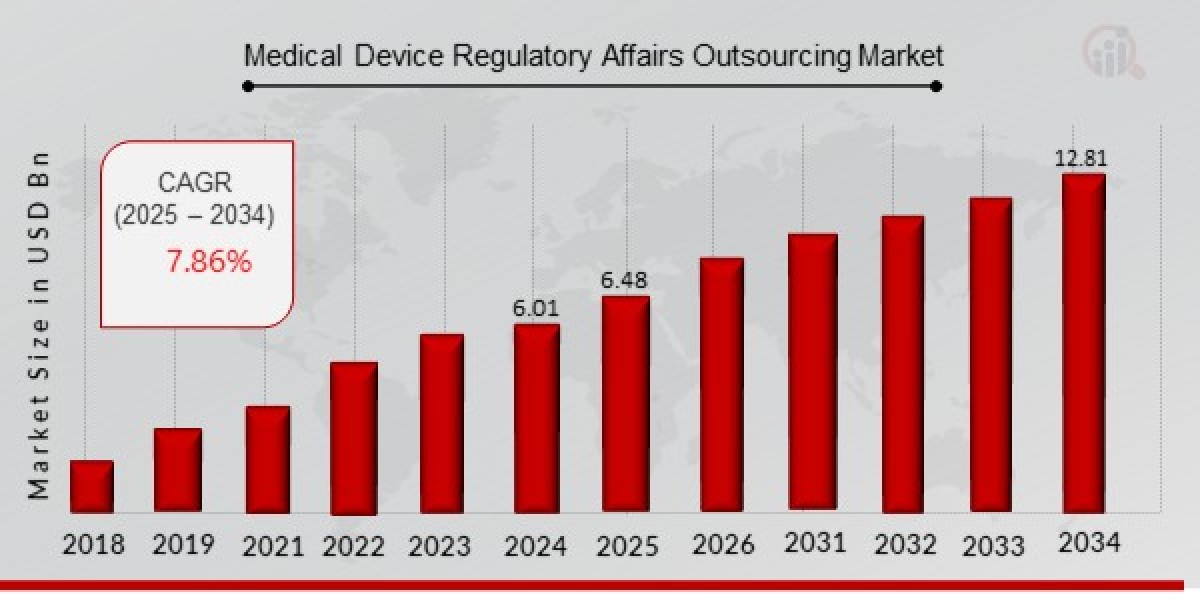
As per MRFR analysis, the Medical Device Regulatory Affairs Outsourcing Market Size was estimated at 6.01 (USD Billion) in 2024. The Medical Device Regulatory Affairs Outsourcing Market Industry is expected to grow from 6.48 (USD Billion) in 2025 to 12.81 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 7.86% during the forecast period (2025 - 2034).
Challenges in Medical Device Regulatory Compliance
Despite the benefits of outsourcing, medical device regulatory affairs still face challenges, such as:
- Frequent Regulatory Changes: Agencies like the FDA and EMA update guidelines, requiring companies to adapt quickly.
- Stringent Data Requirements: Robust clinical data and risk assessment reports are essential for approval.
- Harmonization Across Markets: Navigating different global regulations can be complex.
- Data Security and Confidentiality: Ensuring protection of proprietary data when working with third-party vendors.
Future Trends in Regulatory Affairs Outsourcing
The medical device industry continues to evolve, and regulatory outsourcing is adapting to new trends:
- AI and Automation in Compliance: Leveraging AI-driven tools for regulatory documentation and submission processes.
- Increased Focus on Cybersecurity Compliance: As medical devices become more connected, regulations around data security are tightening.
- Personalized and Digital Health Regulations: With the rise of wearable devices and personalized medicine, regulatory frameworks are adapting.
- Outsourcing to Emerging Markets: Companies are looking beyond traditional regulatory firms, partnering with experts in Asia-Pacific and Latin America.
Final Thoughts
Navigating regulatory compliance in the medical device industry is complex but essential. Medical device regulatory affairs outsourcing enables manufacturers to streamline approval processes, reduce costs, and ensure compliance while focusing on innovation. As the industry grows, leveraging expert regulatory partners will continue to be a game-changer for companies aiming for global success.
For more information, please visit @marketresearchfuture