Choroideremia Market Report Overview:
| Report Attribute | Details |
| Base Year | 2022 |
| Forecast Years | 2023-2033 |
| Historical Years | 2017-2022 |
| Market Growth (2023-2033) | 4.4% |
The report offers a comprehensive analysis of the choroideremia market in the United States, EU5 (including Germany, Spain, Italy, France, and the United Kingdom), and Japan. It covers aspects such as treatment methods, drugs available in the market, drugs in development, the proportion of various therapies, and the market’s performance in the seven major regions. Additionally, the report evaluates the performance of leading companies and their pharmaceutical products. Current and projected patient numbers across these key markets are also detailed in the report. This study is essential for manufacturers, investors, business planners, researchers, consultants, and anyone interested or involved in the choroideremia market.
Request for a Sample Copy of this Report: https://www.imarcgroup.com/choroideremia-market/requestsample
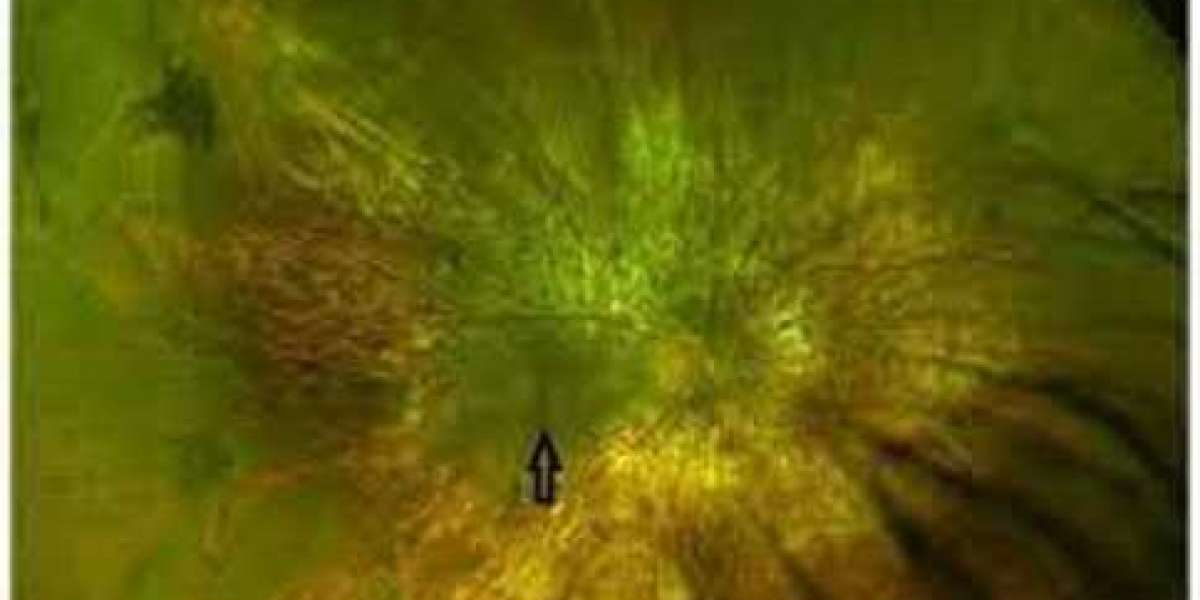
The choroideremia market is expected to exhibit a CAGR of 4.4% during 2023-2033. Choroideremia, a rare genetic eye disorder that leads to progressive vision loss, has seen significant advancements in research and treatment options in recent years. Several key drivers are propelling growth in the choroideremia market, providing hope for patients and investors alike. A growing awareness of choroideremia among healthcare professionals and the general public has led to earlier diagnoses. Timely detection allows for timely intervention and treatment, potentially slowing down disease progression. Gene therapy is gaining popularity as a promising medication approach for choroideremia. Ongoing research and development efforts in gene therapy have yielded positive results, with various clinical trials showing significant improvements in vision among patients. Choroideremia has received orphan drug designation in several regions, providing incentives for pharmaceutical companies to invest in RD activities. This designation offers market exclusivity and tax incentives, encouraging innovation in treatment options. Collaboration among pharmaceutical companies, academic institutions, and patient advocacy groups has accelerated research efforts.
These collaborations foster knowledge exchange and the pooling of resources to expedite the emergence of effective medication. The patient-centric approach to drug development has gained momentum. Patient advocacy groups play a pivotal role in advocating for choroideremia RD and facilitating patient participation in clinical trials. Regulatory agencies, including the FDA and EMA, have shown a willingness to work closely with drug developers to streamline the approval process for potential choroideremia treatments, reducing time to market. Positive clinical trial outcomes and a growing patient population have attracted investor interest. This influx of funding is supporting research, clinical trials, and commercialization efforts. Furthermore, the escalating adoption of low-intensity laser techniques, since they help in preventing retinal cell degeneration as well as slowing down the disease progression, is anticipated to propel the growth of the choroideremia market in the coming years.
Countries Covered:
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country:
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the choroideremia market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the choroideremia market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report also provides a detailed analysis of the current choroideremia marketed drugs and late-stage pipeline drugs.
In-Market Drugs:
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs:
- Drug overview
- Mechanism of action
- Regulatory status
- Clinical trial results
- Drug uptake and market performance
Competitive Landscape With Key Players:
The competitive landscape of the choroideremia market has been studied in the report with the detailed profiles of the key players operating in the market.
Ask Analyst for Customization and Explore Full Report With TOC List of Figures: https://www.imarcgroup.com/request?type=reportid=9744flag=C
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Media Contact:
Company Name: IMARC Group
Contact Person: Elena Anderson
Email: sales@imarcgroup.com
Phone: +1-631-791-1145
Address: 134 N 4th St
City: Brooklyn
State: NY
Country: United States
Website: https://www.imarcgroup.com/