Blood-Brain Barrier Technologies Market
Market Overview
The blood-brain barrier (BBB) technologies market is gaining traction as advancements in neurological disorder treatment drive demand for innovative drug delivery methods. The BBB is a highly selective permeability barrier that prevents harmful substances from entering the brain while allowing essential nutrients to pass through. However, this natural defense also poses a significant challenge for drug delivery in neurological conditions such as Alzheimer's disease, Parkinson’s disease, brain tumors, and multiple sclerosis. As a result, companies are investing in disruptive BBB technologies, including nanoparticle-based drug carriers, ultrasound-mediated delivery, and receptor-mediated transcytosis, to enhance therapeutic efficacy.
Market Size and Share
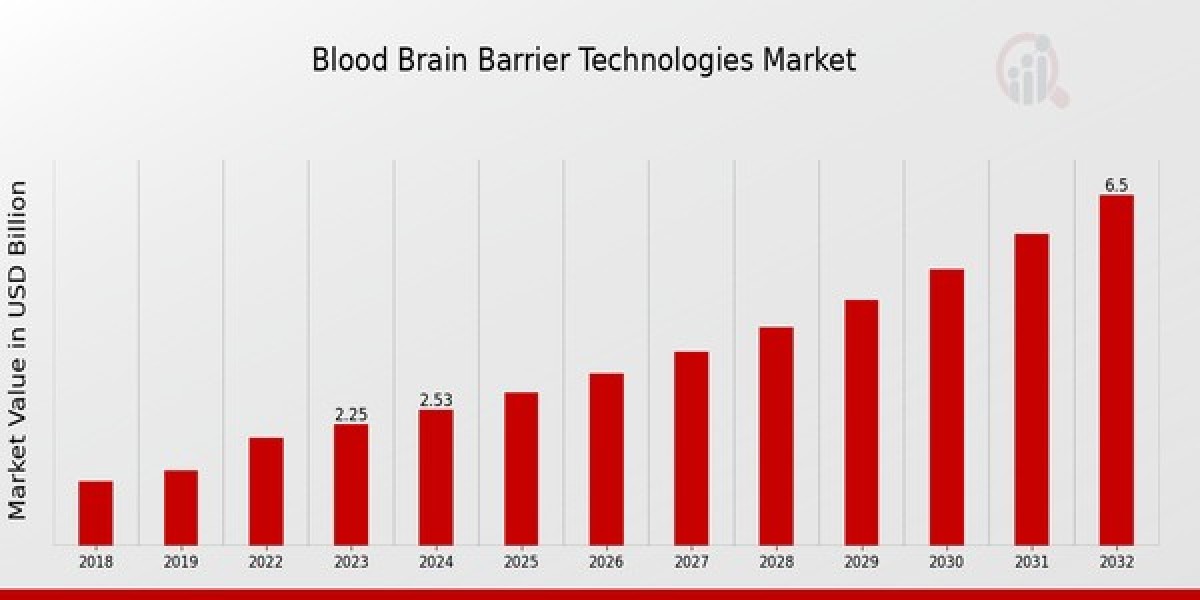
Blood brain barrier technologies market size was estimated at 1.4(USD Billion) in 2023. The blood brain barrier technologies industry is expected to grow from 1.76(USD Billion) in 2024 to 9.85(USD Billion) by 2032. The blood brain barrier technologies market CAGR (growth rate) is expected to be around 21.12% during the forecast period (2024-2032). The global BBB technologies market is experiencing rapid growth, driven by rising cases of neurodegenerative diseases, increasing research funding, and advancements in biopharmaceuticals. North America holds the largest market share due to high R&D investment and strong regulatory support, followed by Europe. Asia-Pacific is emerging as a key player due to the expansion of biotech research and healthcare infrastructure.
Market Trends
- Rising Demand for Non-Invasive BBB Disruption Methods: Techniques like focused ultrasound (FUS) and microbubble-mediated BBB opening are revolutionizing drug delivery for brain disorders.
- Nanotechnology in Drug Transport: Lipid nanoparticles (LNPs) and polymer-based carriers are gaining popularity for targeted drug delivery.
- Growth in Gene and Cell Therapy: BBB-targeted gene therapies are being developed for conditions like glioblastoma and rare neurological diseases.
- Collaborations Between Pharma and Biotech Companies: Strategic partnerships are accelerating clinical research in BBB penetration strategies.
Growth Drivers
- Increasing Prevalence of Neurological Disorders: Conditions such as Alzheimer’s, stroke, and epilepsy are fueling demand for effective brain drug delivery systems.
- Advancements in Biotechnology and Nanomedicine: Artificial intelligence (AI) and CRISPR-based gene editing are enabling more precise BBB penetration techniques.
- Expansion of Personalized Medicine: BBB technologies are being used to develop targeted therapies tailored to genetic profiles of patients.
- Rising Government and Private Research Investments: Increased funding for neuropharmaceutical research is accelerating innovation in BBB transport mechanisms.
Challenges and Restraints
- Complexity of the Blood-Brain Barrier: The highly selective nature of the BBB limits the efficacy of traditional drug delivery methods.
- High Cost of R&D: Developing BBB-penetrating drugs and nanocarriers requires extensive clinical trials and regulatory approvals.
- Limited Success in Clinical Translation: Despite promising research, many BBB-targeting therapies fail to achieve commercial success due to safety concerns.
Regional Analysis
- North America: Dominates the market due to high neurological disorder burden, strong R&D funding, and advanced healthcare infrastructure.
- Europe: Increasing demand for innovative CNS treatments and rising biotech collaborations.
- Asia-Pacific: Rapidly growing market with expanding pharmaceutical research capabilities and government funding for neurological research.
Segmental Analysis
- By Technology:
- Nanoparticle Drug Delivery Systems
- Ultrasound & Microbubble-Assisted BBB Disruption
- Trojan Horse Drug Delivery
- Receptor-Mediated Transcytosis
- By Application:
- Neurodegenerative Diseases (Alzheimer’s, Parkinson’s, Multiple Sclerosis)
- Brain Tumors and CNS Cancer
- Neurovascular Disorders (Stroke, Epilepsy)
Key Market Players
- Denali Therapeutics
- CarThera
- Fujifilm Diosynth Biotechnologies
- Eisai Co., Ltd.
- Bioasis Technologies Inc.
Recent Developments
- Breakthroughs in Nanomedicine for BBB Penetration: Researchers are developing biodegradable nanoparticle drug carriers to cross the BBB safely.
- Clinical Trials for Ultrasound-Based BBB Opening: Non-invasive ultrasound technologies are entering phase II and III clinical trials for conditions like glioblastoma.
- AI-Driven BBB Drug Discovery: Artificial intelligence and machine learning are being used to predict optimal drug formulations for BBB crossing.
For more information, please visit us at @marketresearchfuture.