Market Overview
The BCMA (B-cell maturation antigen) targeted therapy market is rapidly expanding due to the increasing prevalence of multiple myeloma and advancements in immunotherapy-based treatments. BCMA, a protein found on malignant plasma cells, plays a crucial role in the survival and proliferation of myeloma cells. Targeting BCMA has become a breakthrough approach in treating relapsed or refractory multiple myeloma (RRMM), leading to the development of novel therapies, including CAR-T cell therapy, antibody-drug conjugates (ADCs), and bispecific antibodies.
Market Size and Growth Potential
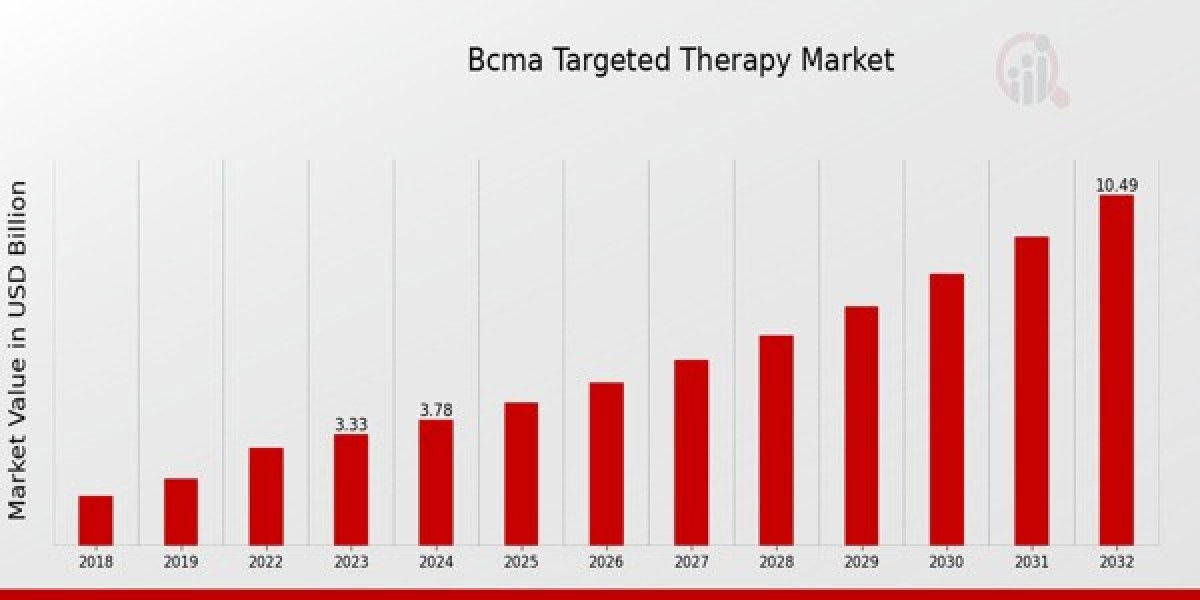
BCMA Targeted Therapy Market Size was estimated at 4.30 (USD Billion) in 2024. The BCMA Targeted Therapy Market Industry is expected to grow from 4.88 (USD Billion) in 2025 to 15.41 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 13.62% during the forecast period (2025 - 2034).
The global BCMA targeted therapy market is witnessing exponential growth due to:
- Rising incidence of multiple myeloma and plasma cell disorders.
- Growing approvals of BCMA-directed therapies by regulatory agencies.
- Increased investment in cell and gene therapy research.
- High demand for personalized and targeted cancer treatment approaches.
Market Trends and Key Developments
- Emergence of BCMA-Targeting CAR-T Cell Therapies:
- FDA-approved BCMA CAR-T therapies like idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti) have revolutionized multiple myeloma treatment.
- Expanding Pipeline of Bispecific Antibodies:
- Drugs like teclistamab show promise in targeting BCMA and CD3 to enhance T-cell mediated myeloma cell destruction.
- Advancements in Antibody-Drug Conjugates (ADCs):
- Belantamab mafodotin (Blenrep) is an ADC that delivers a cytotoxic payload directly to BCMA-expressing cells.
- Combination Therapies for Enhanced Efficacy:
- Research is focused on combining BCMA-targeted therapies with immunomodulators (IMiDs), proteasome inhibitors, and steroids to improve patient outcomes.
Growth Drivers
- Rising Prevalence of Multiple Myeloma
- According to the American Cancer Society, approximately 35,000 new cases of multiple myeloma are diagnosed annually in the U.S.
- Aging populations and genetic predisposition contribute to rising incidence rates.
- FDA Approvals and Regulatory Support
- The approval of BCMA-targeted CAR-T therapies and ADCs has increased patient access to innovative treatments.
- Advancements in Cell Therapy Manufacturing
- Improved CAR-T cell production processes and automation are making therapies more scalable and cost-effective.
- Growing Investment in Cancer Immunotherapy
- Pharmaceutical giants are investing heavily in BCMA-targeted research and development to improve efficacy and reduce side effects.
Challenges and Restraints
- High Cost of BCMA CAR-T Cell Therapies:
- Treatments like Abecma and Carvykti cost over $400,000 per dose, making affordability a significant concern.
- Limited Availability of Advanced Treatment Centers:
- CAR-T therapy requires specialized manufacturing and treatment facilities, limiting its global adoption.
- Potential Side Effects:
- Cytokine release syndrome (CRS) and neurotoxicity are major adverse effects associated with BCMA-targeted therapies.
Regional Analysis
- North America:
- Leads the market due to high cancer prevalence, strong R&D funding, and early regulatory approvals.
- Europe:
- Increasing adoption of BCMA CAR-T and bispecific antibodies is driving growth.
- Asia-Pacific:
- Rising cases of multiple myeloma and increasing healthcare expenditure are propelling market expansion.
Segmental Analysis
- By Therapy Type:
- CAR-T Cell Therapy (Idecabtagene Vicleucel, Ciltacabtagene Autoleucel)
- Antibody-Drug Conjugates (ADCs) (Belantamab Mafodotin)
- Bispecific Antibodies (Teclistamab)
- Monoclonal Antibodies and Other Immunotherapies
- By End-User:
- Hospitals and Cancer Treatment Centers
- Specialty Clinics
- Research Institutions
Key Market Players
- Bristol-Myers Squibb (Abecma)
- Janssen Pharmaceuticals (Carvykti, Teclistamab)
- GSK (Belantamab Mafodotin)
- Regeneron Pharmaceuticals
- Legend Biotech Corporation
- Pfizer Inc.
Recent Developments
- Expanded Use of BCMA CAR-T Therapy in Early Myeloma Treatment
- Strategic Collaborations Between Pharma Companies for BCMA Drug Development
- Launch of Next-Generation BCMA-Targeting Antibody Therapies
Future Outlook
The BCMA targeted therapy market is expected to witness continued advancements in next-generation immunotherapies, enhanced manufacturing capabilities, and broader patient accessibility. As combination treatments and off-the-shelf CAR-T therapies gain traction, the market is poised for strong expansion in the coming years.
For more information, please visit us at @marketresearchfuture.