Introduction: A Race Against Time for Vision Loss
Imagine slowly losing your sight, knowing there’s no cure—yet. This is the reality for individuals with choroideremia, a rare, inherited retinal disease that causes progressive vision loss. Affecting mostly men, this condition gradually erodes the retina’s ability to function, ultimately leading to blindness.
But a breakthrough in gene therapy is offering newfound hope. Scientists are harnessing the power of genetic medicine to halt, or even reverse, vision loss in choroideremia patients. Could gene therapy be the game-changer the world has been waiting for?
Understanding Choroideremia: What Goes Wrong?
Choroideremia is caused by mutations in the CHM gene, responsible for producing Rab escort protein-1 (REP-1). Without this protein, cells in the retina's choroid layer degenerate over time, leading to:
? Night blindness (one of the first symptoms)
? Loss of peripheral vision (tunnel vision)
? Complete blindness (in advanced stages)
Since the disease is X-linked, it primarily affects males, while females often carry the mutation without experiencing full vision loss.
Gene Therapy: The Dawn of a New Era
For years, doctors could only manage symptoms, but gene therapy is now offering a potential one-time treatment. The approach involves:
- Delivering a healthy CHM gene via an adeno-associated virus (AAV) into retinal cells.
- Allowing the retina to produce REP-1, preventing further degeneration.
- Preserving or restoring vision, offering long-term benefits.
Unlike traditional treatments that slow disease progression, gene therapy addresses the root cause, making it a revolutionary approach.
Breakthrough Clinical Trials: What’s the Progress?
Recent trials have shown promising results, with many patients experiencing stabilized vision or even slight improvements. Some key findings include:
✔️ Sustained vision for over 5 years post-treatment
✔️ Increased retinal sensitivity
✔️ No severe side effects observed
While more research is needed to refine this therapy, the results so far indicate a potential cure is within reach.
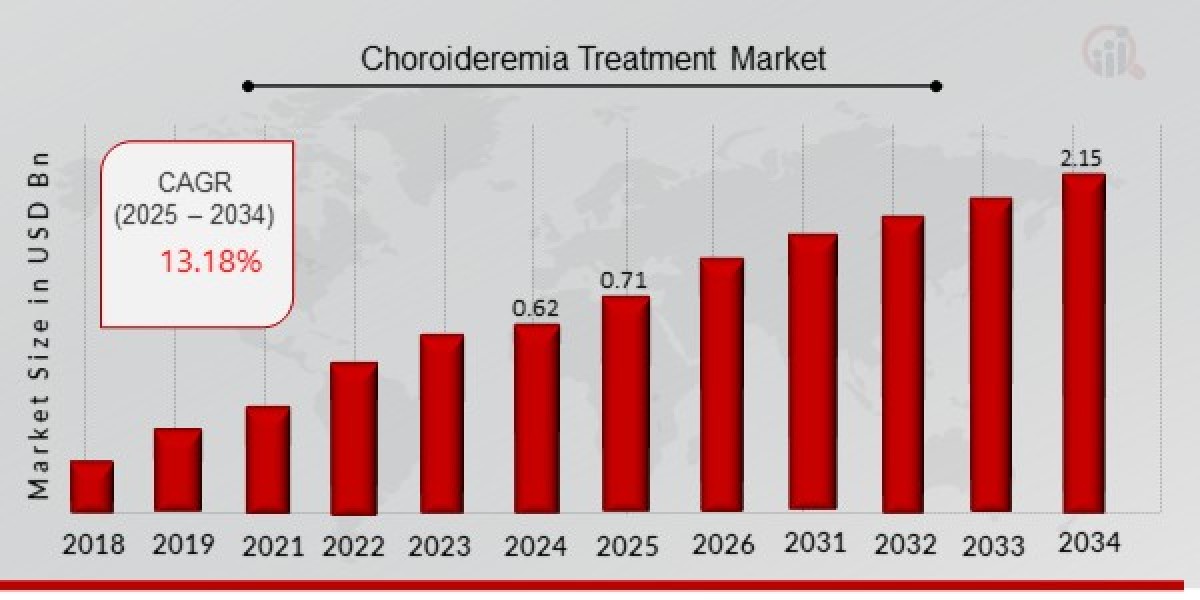
Choroideremia Treatment Market Overview
As per MRFR analysis, the Choroideremia Treatment Market Size was estimated at 0.62 (USD Billion) in 2024. The Choroideremia Treatment Market Industry is expected to grow from 0.71 (USD Billion) in 2025 to 2.15 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 13.18% during the forecast period (2025 - 2034).
Challenges and the Road Ahead
Despite the progress, gene therapy for choroideremia still faces hurdles:
? Long-term durability – How long will the effects last?
? Optimal delivery methods – How can we ensure the best uptake of the new gene?
? Cost & accessibility – Will this treatment be available to all who need it?
Scientists are actively working on new viral vectors, safer gene-editing techniques, and affordable treatment options to overcome these challenges.
A Hopeful Future
Gene therapy is ushering in a new era of medicine, where rare genetic diseases like choroideremia may no longer be a lifelong sentence. With continued advancements, blindness from inherited retinal diseases could soon become a thing of the past.
Conclusion
For choroideremia patients, gene therapy represents more than just treatment—it’s a beacon of hope. As ongoing research refines this revolutionary approach, the dream of preventing blindness is closer than ever before.
For more information, please visit @marketresearchfuture